Pustular Eruptions in Children as Manifestations of Autoinflammatory Diseases
Abstract
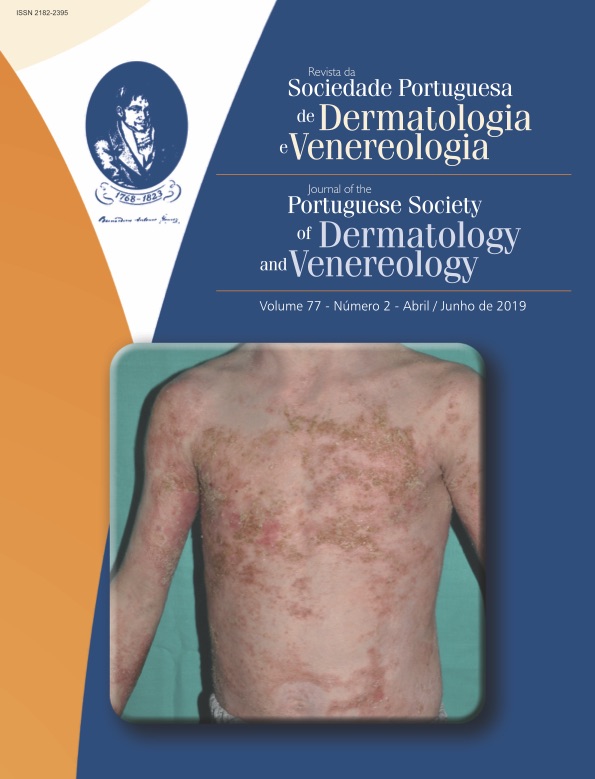
Nowadays, in clinical practice, when attending a child with a pustular eruption and systemic inflammation, it is mandatory to think of an autoinflammatory disease, once infectious causes have been ruled out. Although rare, autoinflammatory disease must be recognized as early as possible, accurately diagnosed (including gene testing), and treated with targeted therapy if available.
Downloads
References
Ben-Chetrit E, Gattorno M, Gul A, Kastner DL, Lachmann HJ, Touitou I, et al. Consensus proposal for taxonomy and definition of the autoinflammatory diseases (AIDs): a Delphi study; Paediatric Rheumatology International Trials Organisation (PRINTO) and the AIDs Delphi study participants. Ann Rheum Dis. 2018;77:1558-1565.
Russo RA, Brogan PA. Monogenic autoinflammatory diseases. Rheumatology (Oxford). 2014;53:1927-39.
Moghaddas F, Masters SL. The classification, genetic diagnosis and modelling of monogenic autoinflammatory disorders. Clinical Science. 2018;132:1901–1924.
Almeida de Jesus A, Goldbach-Mansky R. Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin Immunol. 2013;147:155-74.
Hernández-Ostiz S, Prieto-Torres L, Xirotagaros G, Noguera-Morel L, Hernández-Martín Á, Torrelo A. Autoinflammatory Diseases in Pediatric Dermatology-Part 1: Urticaria-like Syndromes, Pustular Syndromes, and Mucocutaneous Ulceration Syndromes. Actas Dermosifiliogr. 2017;108:609-619.
Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-25.
Dávila-Seijo P, Hernández-Martín A, Torrelo A. Autoinflammatory syndromes for the dermatologist. Clin Dermatol. 2014;32:488-501.
Marzano AV, Damiani G, Genovese G, Gattorno M. A dermatologic perspective on autoinflammatory diseases. Clin Exp Rheumatol. 2018;36:32-38.
Posso-De Los Rios CJ, Pope E. New insights into pustular dermatoses in pediatric patients. J Am Acad Dermatol. 2014;70:767-773.
Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-25.
Aksentijevich I, Masters SL, Ferguson PJ, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426-37.
Mendonca LO, Malle L, Donovan FX, et al. Deficiency of Interleukin-1 Receptor Antagonist (DIRA): Report of the First Indian Patient and a Novel Deletion Affecting IL1RN. J Clin Immunol. 2017;37:445-451.
Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-52.
Schnellbacher C, Ciocca G, Menendez R, Aksentijevich I, Goldbach-Mansky R, Duarte AM, et al. Deficiency of interleukin-1 receptor antagonist responsive to anakinra. Pediatr Dermatol. 2013;30:758-60.
Brau-Javier CN, Gonzales-Chavez J, Toro JR. Chronic cutaneous pustulosis due to a 175-kb deletion on chromosome 2q13: excellent response to anakinra. Arch Dermatol. 2012;148:301-4.
Majeed, HA, Al-Tarawna, M, El-Shanti, H, et al. The syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia. Report of a new family and a review. European Journal of Pediatrics. 2001;160:705–710.
Rao AP, Gopalakrishna DB1, Bing X, Ferguson PJ. Phenotypic Variability in Majeed Syndrome. J Rheumatol. 2016;43:1258-9.
Ferguson PJ, Chen S, Tayeh MK, et al. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome). J Med Genet. 2005;42:551-7.
Pinto-Fernández C, Seoane-Reula ME. Efficacy of treatment with IL-1RA in Majeed syndrome. Allergol Immunopathol (Madr). 2017;45:99-101.
Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH Syndromes: Pathophysiology, Presentation and Treatment. Am J Clin Dermatol. 2017;18:555-562.
Demidowich AP, Freeman AF, Kuhns DB, Aksentijevich I, Gallin JI, Turner ML, et al. Brief report: genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne). Arthritis Rheum 2012;64:2022-7.
Lee H, Park SH, Kim SK, Choe JY, Park JS. Pyogenic arthritis, pyoderma gangrenosum, and acne syndrome (PAPA syndrome) with E250K mutation in CD2BP1 gene treated with the tumor necrosis factor inhibitor adalimumab. Clin Exp Rheumatol 2012;30:452.
Stichweh DS, Punaro M, Pascual V. Dramatic improvement of pyoderma gangrenosum with infliximab in a patient with PAPA syndrome. Pediatr Dermatol 2005;22:262-5.
Dierselhuis MP, Frenkel J, Wulffraat NM, Boelens JJ. Anakinra for flares of pyogenic arthritis in PAPA syndrome. Rheumatology (Oxford). 2005;44:406-8.
Braun-Falco M, Kovnerystyy O, Lohse P, Ruzicka T. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) – a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol 2012; 66:409–15.
Marzano AV, Trevisan V, Gattorno M et al. Pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis supurativa (PAPASH): a new autoinflammatory syndrome associated with a novel mutation of the PSTPIP1 gene. JAMA Dermatol 2013;149:762-4.
Vinkel C, Thomsen SF1. Autoinflammatory syndromes associated with hidradenitis suppurativa and/or acne. Int J Dermatol. 2017;56:811-818.
Kotlarz D, Beier R, Murugan D, et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143:347-55.
Zheng C1, Huang Y1, Hu W1, Shi J1, et al. Phenotypic Characterization of Very Early-Onset Inflammatory Bowel Disease with Interleukin-10 Signaling Deficiency: Based on a Large Cohort Study. Inflamm Bowel Dis. 2019;25:756-766.
Marrakchi S, Guigue P, Renshaw BR et al. Interleukin-36- receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med 2011;365:620 8.
Akiyama M, Takeichi T, McGrath JA, Sugiura K. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci 2018;90:105–111.
Cowen EW, Goldbach-Mansky R. DIRA, DITRA, and new insights into pathways of skin inflammation: what's in a name? Arch Dermatol. 2012;148:381-4.
Tauber M, Viguier M, Le Gall C, Smahi A, Bachelez H. Is it relevant to use an interleukin-1-inhibiting strategy for the treatment of patients with deficiency of interleukin-36 receptor antagonist? Br J Dermatol 2014;170:1198-9.
Molho-Pessach V, Alyan R, Gordon D, Jaradat H, Zlotogorski A. Secukinumab for the treatment of deficiency of interleukin 36 receptor antagonist in an adolescent. JAMA Dermatol 2017;153:473-475.
Sugiura K, Endo K, Akasaka T, Akiyama M. Successful treatment with infliximab of sibling cases with generalized pustular psoriasis caused by deficiency of interleukin-36 receptor antagonist. J Eur Acad Dermatol Venereol 2015;29:2054-2056.
Hussain S, Berki DM, Choon SE, Burden AD, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1069.
Cordoro KM1, Ucmak D1, Hitraya-Low M1, Rosenblum MD1, Liao W1. Response to Interleukin (IL)-17 Inhibition in an Adolescent With Severe Manifestations of IL-36 Receptor Antagonist Deficiency (DITRA). JAMA Dermatol. 2017;153:106-108.
Bonekamp N, Caorsi R, Viglizzo GM, Graaf M, Minoia F, Grossi A, et al. High-dose ustekinumab for severe childhood deficiency of interleukin-36 receptor antagonist (DITRA). Ann Rheum Dis 2018;77:1241-1243.
Rossi-Semerano L, Piram M, Chiaverini C, De Ricaud D, Smahi A, Koné-Paut I. First clinical description of an infant with interleukin-36-receptor antagonist deficiency successfully treated with anakinra. Pediatrics. 2013;132:1043-1047.
Jordan CT, Cao L, Roberson ED, Pierson KC, Yang CF, Joyce CE, et al. PSORS2 is due to mutations in CARD14. Am J HumGenet 2012;90:784-95.
Craiglow BG, Boyden LM, Hu R, et al. CARD14-associated papulosquamous eruption: A spectrum including features of psoriasis and pityriasis rubra pilaris.
J Am Acad Dermatol. 2018;79:487-494.
Berki DM, Liu L, Choon SE, et al. Activating CARD14 Mutations Are Associated with Generalized Pustular Psoriasis but Rarely Account for Familial Recurrence in Psoriasis Vulgaris. J Invest Dermatol. 2015;135:2964-2970.
Takeichi T, Kobayashi A, Ogawa E, Okuno Y, Kataoka S, Kono M, et al. Autosomal dominant familial generalized pustular psoriasis caused by a CARD14 mutation. Br J Dermatol. 2017;177:133-135.
Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-92.
Setta-Kaffetzi N, Simpson MA, Navarini AA, Patel VM, Lu HC, Allen MH, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
Mössner R, Wilsmann-Theis D, Oji V, et al. The genetic basis for most patients with pustular skin disease remains elusive. Br J Dermatol. 2018;178:740-748.
Habal NC, Chen Y, Jordan C, et al. Pathogenesis Study of Infantile-Onset, Severe Pustular Psoriasis Reveals a De Novo Mutation in CARD14 Causing Psoriasis Which Responds Clinically to IL-12/23 Blocking Treatment with Ustekinumab. Arthritis and Rheumatism. 2011:63.
Masters SL, et al. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Science Translational Medicine. 2016;8:332-345.
Moghaddas F, Llamas R, De Nardo D, Martinez-Banaclocha H, Martinez-Garcia JJ, Mesa-Del-Castillo P, et al. A novel Pyrin-Associated Autoinflammation with Neutrophilic Dermatosis mutation further defines 14-3-3 binding of pyrin and distinction to Familial Mediterranean Fever. Ann Rheum Dis. 2017;76:2085-2094.
Gargallo V, Menis D, Delgado Márquez AM, Aróstegui JI, Llamas Martín R. Short-term efficacy of adalimumab in a patient with pyrin-associated autoinflammation with neutrophilic dermatosis. J Dtsch Dermatol Ges. 2018;16:756-759.
Ombrello MJ, Remmers EF, Sun G, Freeman AF, Datta S, Torabi-Parizi P, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. The New England journal of medicine. 2012; 366:330–8.
Aderibigbe OM, Priel DL, Lee CC, Ombrello MJ, Prajapati VH, Liang MG, Lyons JJ, Kuhns DB, Cowen EW, Milner JD. Distinct Cutaneous Manifestations and Cold-Induced Leukocyte Activation Associated With PLCG2 Mutations. JAMA Dermatol. 2015;151:627-34.
Zhou Q, Lee GS, Brady J, Datta S, Katan M, Sheikh A, et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cgamma2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. American journal of human genetics. 2012;91:713–20.
All articles in this journal are Open Access under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC 4.0).